On April 16, Accuray inaugurated its Innovation & Partnership Hub in Genolier, Switzerland, reaffirming its commitment to the radiotherapy field as well as the Canton of Vaud. Positioned strategically within the Genolier Innovation Hub, the new facility capitalizes on the proximity of hospital groups and many investments by the Canton of Vaud making it a region of excellence in the management of benign and malignant tumors. This environment is expected to foster collaborations driving cancer treatment innovation.
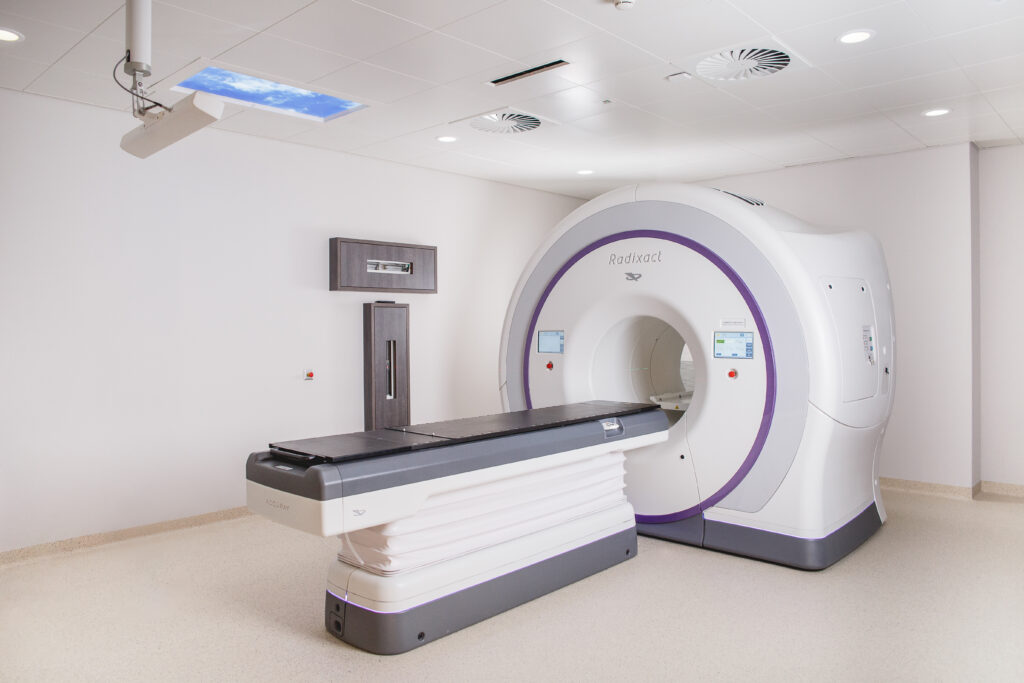
Spanning 500m², the Hub is serving as a crucial training center for Accuray systems renowned for their precision and accuracy in the delivery of radiation. It features today the Radixact® Systems, along with two training rooms and a high-tech studio for online training. In the summer, the CyberKnife® System will be installed.
The event gathered approximately 80 people who enjoyed presentations, a cutting-ribbon ceremony and a guided tour of Accuray facility. We want to thank André Darmon, Mr Le Syndic, City of Genolier, Antoine Hubert, Delegate of the Board of Directors, AEVIS Victoria, Anna Gräbner, CEO of the Genolier Innovation Hub, Prof Oscar Matzinger, Medical Director of the Swiss Radio Oncology Network, and all our guests who honored us with their presence at this crucial milestone in our journey. The support and enthusiasm made this day unforgettable.