The CyberKnife® System: Bringing New Hope for Liver Cancer Patients
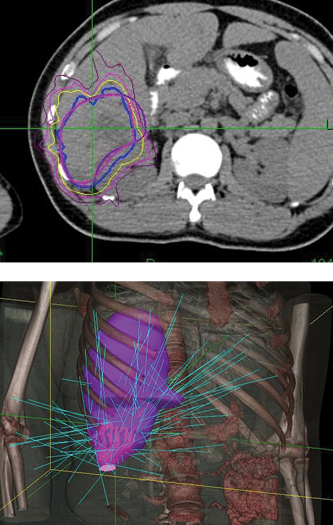
A 42-year-old male with chronic hepatitis B was initially diagnosed with primary liver cancer. He received radiofrequency ablation. Then an MRI revealed recurrence in front of the original lesion. The patient underwent interventional therapy in March 2012, but was hospitalized a month later when a CT showed a residual tumor and acute cholecystitis. Diagnosis was hepatocellular carcinoma, T2N0M0, Stage II. The patient was treated with the CyberKnife® System at 302 Hospital of PLA.
The initial follow-up took place four months after treatment, and CT showed no residual tumor. At the eight-month follow-up, MRI examination showed a region of necrosis in the location of the right lobe tumor. At 32 months post treatment an MRI showed no residual disease or any late toxicities.
“The CyberKnife System’s ability to track and adjust the beam with fiducials is key to giving high-dose SBRT to the liver.”
– Li Yu, M.D., Chairman, Radiation Oncology, 302 Hospital of PLA
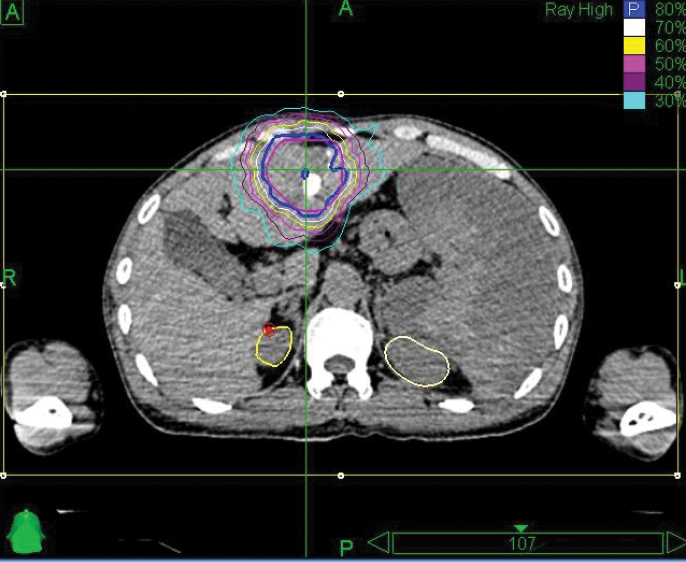
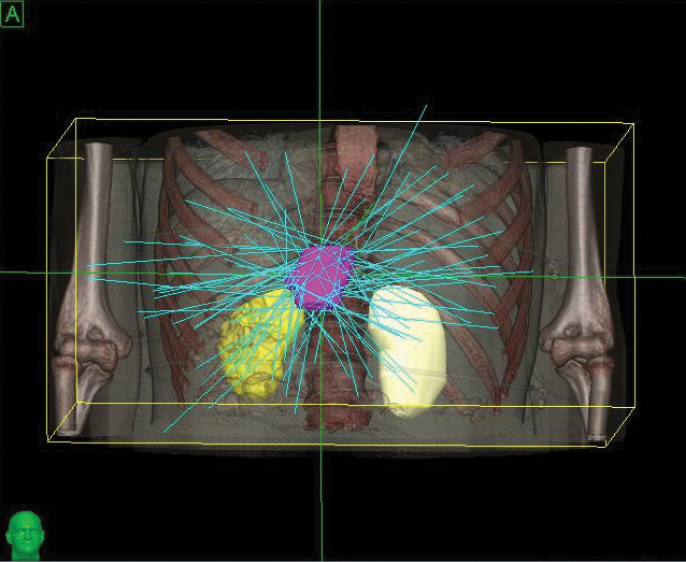
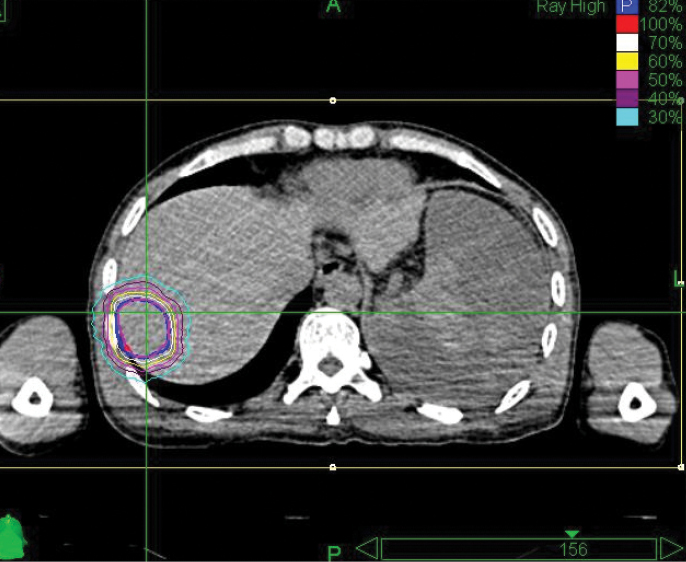
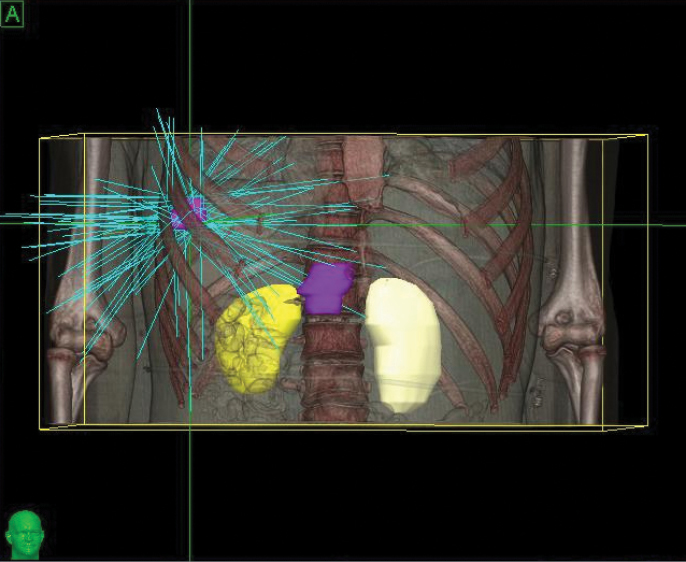
The CyberKnife® System: Extending Life with Precise Treatments
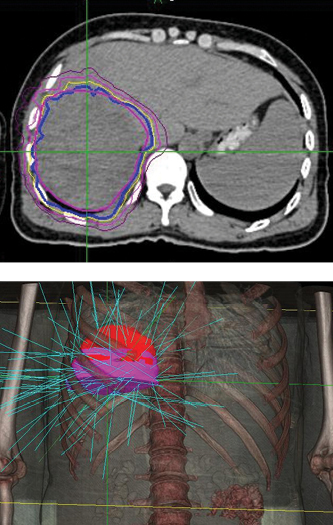
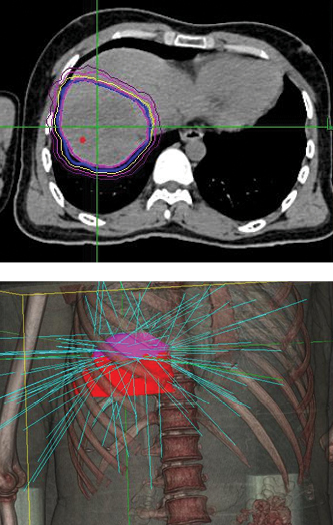
Phase 1 treatment covered the right lower lobe and hilar nodes, divided into two targets. The prescription was 48 Gy, delivered in four 12 Gy fractions for each of the two targets. Bilirubin fluctuations were noted during the course of therapy, but gradually returned to normal after treatment. One month after radiotherapy, a CT scan showed tumor shrinkage. The left lobe had sufficiently enlarged in compensation, so the second stage of treatment was initiated.
Phase 2 treatment covered the right upper lobe, divided into two targets: The prescription was 39 Gy, delivered in three 13 Gy fractions for each of the two targets.
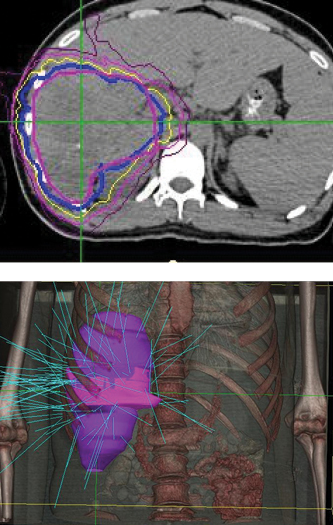
“The patient lived more than two years with good quality of life after initial diagnosis, when life expectancy would have been one month without CyberKnife treatment.”
Important Safety Information
Most side effects of radiotherapy, including radiotherapy delivered with Accuray systems, are mild and temporary, often involving fatigue, nausea, and skin irritation. Side effects can be severe, however, leading to pain, alterations in normal body functions (for example, urinary or salivary function), deterioration of quality of life, permanent injury, and even death. Side effects can occur during or shortly after radiation treatment or in the months and years following radiation. The nature and severity of side effects depend on many factors, including the size and location of the treated tumor, the treatment technique (for example, the radiation dose), and the patient’s general medical condition, to name a few. For more details about the side effects of your radiation therapy, and to see if treatment with an Accuray product is right for you, ask your doctor. Accuray Incorporated as a medical device manufacturer cannot and does not recommend specific treatment approaches. Individual results may vary.
© 2022 Accuray Incorporated. All Rights Reserved. Accuray, the Accuray logo, and other trademarks are trademarks or registered trademarks of Accuray Incorporated and may not be used without permission. For more information on Accuray and its trademarks, please visit www.accuray.com/trademarks.
MKT000609(2)