“Stereotactic body radiation therapy (SBRT) for patients with low and favorable intermediate risk prostate cancer should be considered a new standard of care,” reports Prof. Nicholas van As, Consultant Clinical Oncologist and Medical Director of The Royal Marsden NHS Foundation Trust. The PACE B trial has demonstrated that five-fraction SBRT is non-inferior to conventional radiotherapy regimes in this group of patients. It is a more convenient and cost-effective way to deliver radiotherapy for healthcare providers and patients.
What is PACE?
The PACE (Prostate Advances in Comparative Evidence) study is an international multicenter randomized study of low-, intermediate-, and high-risk localized prostate cancer. Professor Nicholas van As leads the study as principle investigator, which includes three parallel randomization schemes based on patients’ surgery eligibility and risk group. [1]
- PACE-A: Low- and intermediate-risk patients, for whom surgery is a consideration, were randomized to either prostatectomy or prostate SBRT
- PACE-B : Low- and intermediate-risk patients, for whom surgery is not a consideration, were randomized to either standard radiotherapy (conventionally fractionated or moderately hypo-fractionated) or prostate SBRT
- PACE-C: Intermediate- and high-risk patients, for whom androgen deprivation therapy (ADT) treatment was indicated and surgery was not a consideration, were randomized to either conventionally fractionated radiotherapy or prostate SBRT
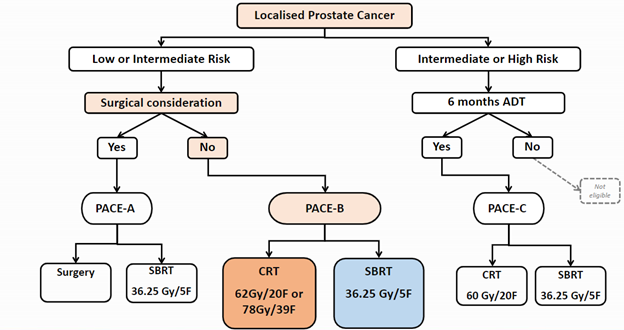
In 2022 a 4th study called PACE-NODES opened. This study compares the safety and efficacy of SBRT to the prostate with SBRT to the prostate and lymph nodes in the pelvis. Both groups receive 5 treatments on alternative days over two weeks. The trial is designed for men with high-risk prostate cancer and recruitment is expected to end in September 2025.[2]
PACE-B trial
How was the trial conducted?
- Between August 2012 and January 2018, 874 patients were recruited to the study from 38 centers in three countries (UK, Ireland, Canada)
- 441 patients were randomized to the control arm of either 78 Gy in 39 fractions or 62 Gy in 20 fractions
- 433 patients were randomized to the SBRT arm, having 36.25 Gy in 5 fractions over 1-2 weeks (daily or alternative days)
- 41% of men in the SBRT arm were treated with the CyberKnife® System
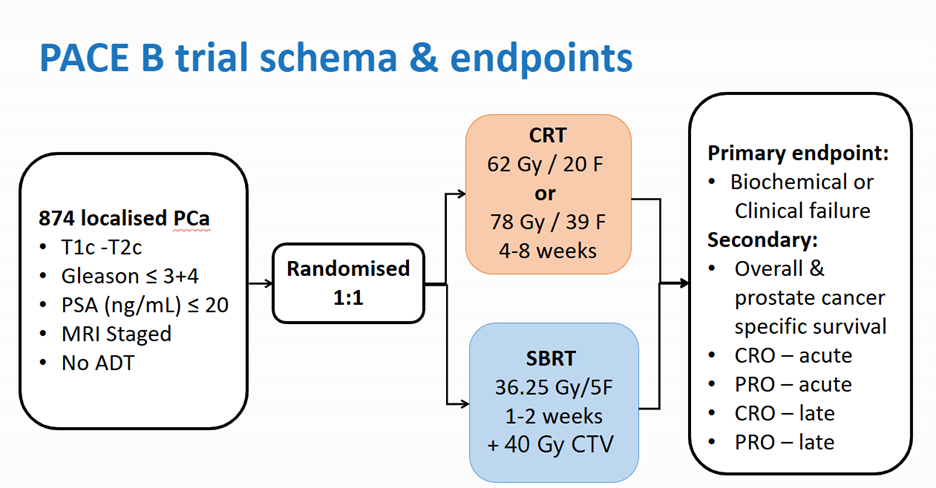
Results
Acute Toxicity
The first results to be published from the PACE-B trial were acute toxicity, that is, side effects experienced by the patient in the first 12 weeks following treatment. This was presented at ASCO GU in February 2019 by Professor Nicholas van As, and published in the Lancet Oncology in November of the same year. They found that acute toxicities were low for both the standard arm and the SBRT arm, and no significant differences were observed between the arms. This was a very promising result as it gave confidence that the higher doses were not causing undesired side effects.[3]
Late Toxicity
The late toxicity results, that is toxicity experienced by patients from 6 months after treatment up to the first 2 years following treatment, were presented at ESTRO 2021 by Dr Alison Tree, Consultant Clinical Oncologist at The Royal Marden NHS Foundation Trust and published in the Lancet Oncology in September, 2022. Once again, the rates of toxicity were low in both arms, and the rates of gastrointestinal (GI) toxicity were comparable. However, when evaluated using the CTCAE scale, the genitourinary (GU) toxicity was found to be higher in the SBRT arm.[4]
When the protocol was amended to allow the inclusion of C-arm linacs in 2014, the team decided that in the 2-year toxicity assessment a comparison would be performed between patients in the SBRT arm treated with the CyberKnife System and those receiving SBRT using a conventional linac. They found that patients receiving treatment with the CyberKnife System experienced less frequent CTCAE (common terminology criteria for adverse events) GU and GI toxicity than those patients treated with conventional linacs. CTCAE GU grade 2 or worse toxicity occurred in 6% of patients treated on the CyberKnife System compared to 17% of patients treated on C-arm linacs. CTCAE GI grade 2 or worse occurred in 1% of patients treated on the CyberKnife System verses 6% on C-arm linacs. No differences were found in RTOG (radiation therapy oncology group) GU and GI toxicities. The toxicity experienced by patients treated with SBRT using the CyberKnife System was no different to the toxicity experienced by patients treated with standard fractionation at those centers with CyberKnife Systems.[4]
The CTCAE GI and GU toxicity up to 5 years showed no significant differences between conventional radiotherapy and SBRT.
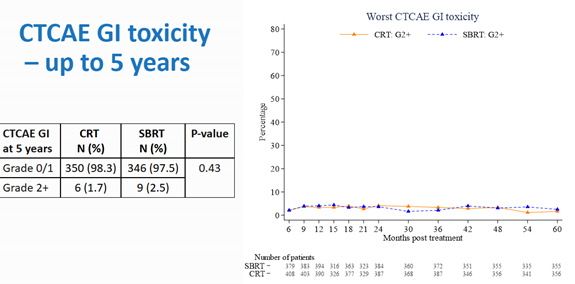
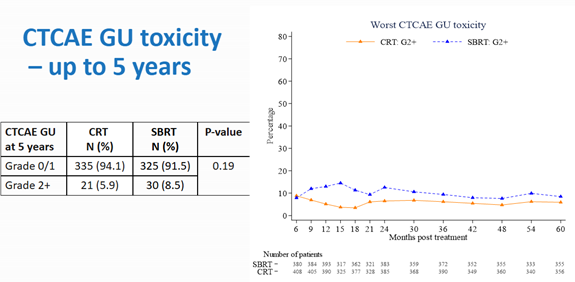
This study provided level one evidence supporting the efficacy of SBRT in low-risk and intermediate-risk prostate cancer, based on a large number of patients. It was the first phase 3 trial reporting late toxicity results at 2 years. The key take away was that toxicity was low for both conventional and SBRT groups regardless of fractionation schedules.
Biochemical Outcomes at 5 years
The announcement that the community was waiting for finally came at ASTRO 2023. To a packed auditorium Prof. Nick van As shared the primary outcome of the study, the biochemical outcomes at 5 years. The data showed that 5-year biochemical/clinical failure free-rate was 95.8% for the SBRT arm, and 94.6% for the conventional arm and the statistical analysis revealed that this was statistically significant for non-inferiority. Put simply, Prof. Nick van As concluded that treatment with 5-fraction SBRT had equivalent efficacy and enhanced efficiency compared to treatment of low-risk and intermediate-risk prostate cancer as the longer fractionation schedule. The paper was published in the New England Journal of Medicine in October.[5]
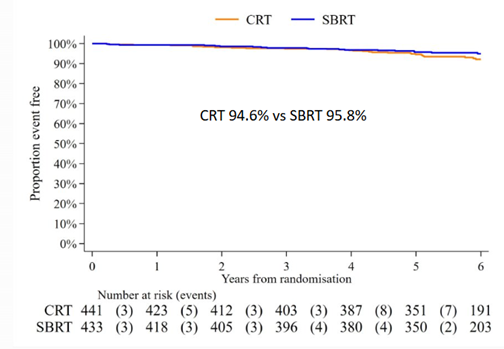
Conclusion
The ability to treat low- or intermediate-risk localized prostate cancer patients in 5 fractions instead of 20 or 39 will make a significant difference to patients’ quality of life. In addition, at a time where hospital resources are so stretched, this will help to alleviate the burden on healthcare systems. The high 5-year biochemical control rates and the advancements in radiotherapy underscore the potential of SBRT use in prostate cancer treatment. It is expected that 5-fraction SBRT will be adopted in the UK very quickly for suitable patients.
References:
- Prostate Advances in Comparative Evidence (PACE) https://www.clinicaltrials.gov/study/NCT01584258
- A trial looking at stereotactic body radiotherapy for prostate cancer (PACE-NODES) | Cancer Research UK
- Brand, D et al. ’Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomized, open-label, phase 3, non-inferiority trial.’ The Lancet Oncology, Volume 20, Issue 11, 1531 – 1543
- Tree AC et al. ‘PACE Trial Investigators. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomized, phase 3, non-inferiority trial.’ Lancet Oncol. 2022 Oct;23(10):1308-1320. doi: 10.1016/S1470-2045(22)00517-4. Epub 2022 Sep 13. Erratum in: Lancet Oncol. 2023 May;24(5):e192. doi: 10.1016/S1470-2045(23)00177-8. PMID: 36113498.
- van As N et al. ‘Phase 3 Trial of Stereotactic Body Radiotherapy in Localized Prostate Cancer.’ N Engl J Med. 2024 Oct 17;391(15):1413-1425. doi: 10.1056/NEJMoa2403365. PMID: 39413377; PMCID: PMC7616714.